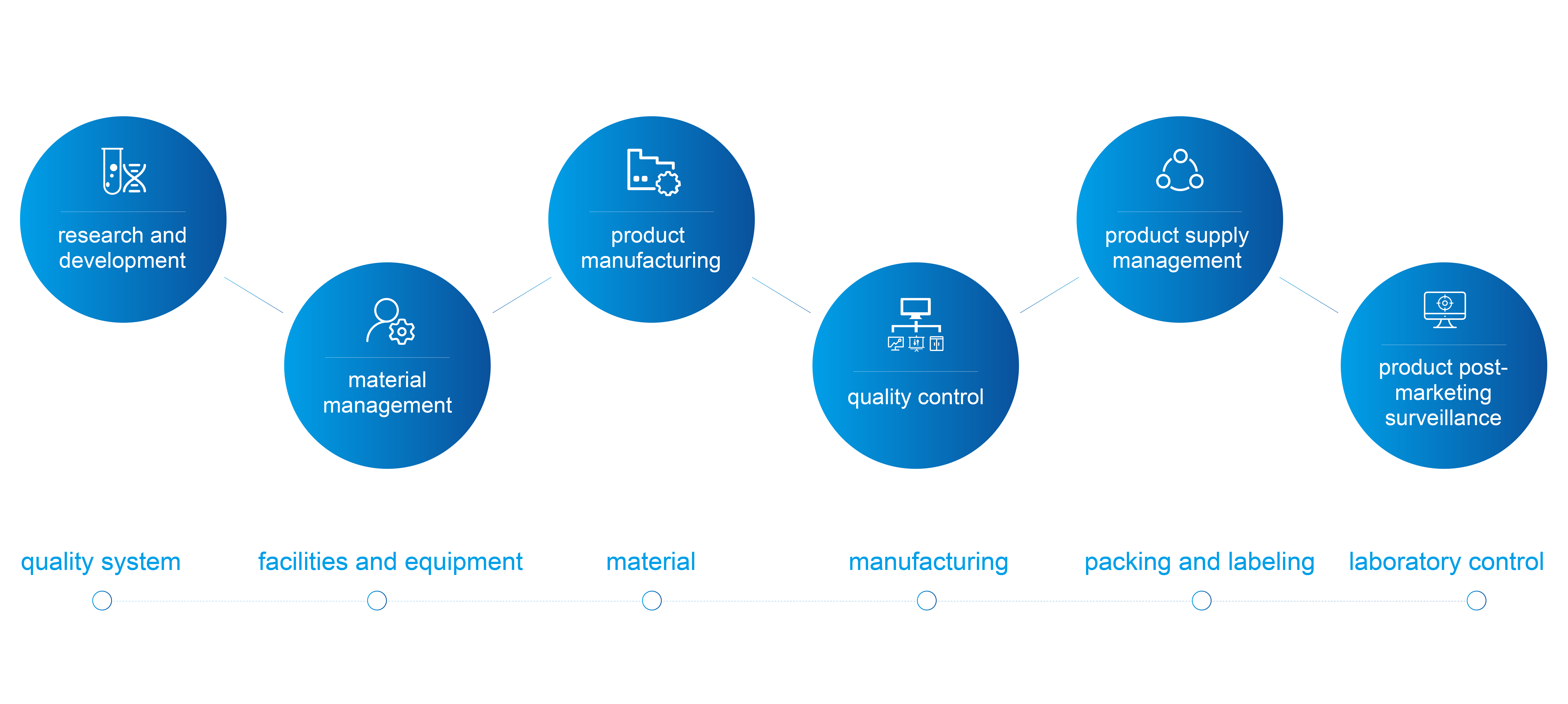
Ever since the incorporation, Henlius has established a quality management system in line with international quality standards, which covers the entire product life cycle, from research and development to material management, product manufacturing, quality control, product supply management and particularly, product post-marketing surveillance, laying the foundation for globalisation.
Xuhui Facility, certificated by China and the EU GMP, has also passed on-site inspections and audits conducted by the NMPA, EMA, the European United (EU) QP and international commercial partners such as Accord and Cipla. Songjiang First Plant has obtained Drug Manufacturing Certificate in 2021, passed GMP compliance on-site inspection and received QP declaration of its GMP equivalence to EU GMP in 2022. It is also eyeing passing the FDA GMP inspection in 2023. Songjiang Second Plant, which is currently under construction, also strictly follows international GMP standards, adopts new technologies to ensure quality and reduce cost, and meets the international level of biomedical automation, informatization, and intelligence (Pharmaceutical Industry 4.0).
Led by a quality management team rich in international working experience and underpinned by the “Quality-centric Culture”, Henlius will continue to redouble efforts in improving quality management system and operation efficiency.